Relying on its own high-level biosafety laboratory construction experience and a strong technical team, KOMAN has established a set of strict high-level biosafety laboratory construction full-process services, from planning and design, material selection to construction, testing and acceptance, strictly Quality control, strive to provide strong technical support for front-line prevention and control treatment, create a safe experimental environment for the development of China's medicine and biotechnology, prevent and resolve laboratory biosafety risks, and contribute to improving the level of biosafety protection in China!
High-level biosafety laboratory design and construction integration
KOMAN company has strong professional configuration, has deep experience in ventilation, electromechanical, control, process and other majors, and has strong technical support for P3 laboratory in process design, HVAC design, electromechanical design, etc.
The modular assembly construction method can be more scientific and efficient during the design and construction of the P3 laboratory, and is more conducive to later operation and maintenance.
KOMAN has a wealth of big data research support in the biomedical field, and has rich construction experience. Customers come from high-level biosafety laboratories, pharmaceutical companies, and medical institutions across the country.
Our country's construction situation
A total of about 127 high-level biosafety laboratories have been built, including 72 from the Health and Family Planning Commission (including 1 P4); 24 from the Ministry of Agriculture (including 1 P4); 8 from the General Administration of Quality Supervision, Inspection and Quarantine, and 23 (including 1 P4). 1 P4); a total of 56 accredited and operated by CNAS, as well as mobile biosafety laboratories; about 30 CDC systems, about 10 scientific research institutions or universities, and several entry-exit inspection and quarantine institutions; distribution Among the 16 provinces and cities, most of them are concentrated in the east and southeast regions, only 5 are in the central provinces and 4 are in the western provinces; Beijing is the most concentrated, 18, followed by Shanghai, Guangdong, Wuhan and other places; related departments; Development and Reform Commission and Ministry of Science and Technology (project establishment), Ministry of Environmental Protection (environmental assessment), Ministry of Health and Construction, Ministry of Agriculture (competent department), Ministry of Housing and Urban-Rural Development (standard formulation and acceptance)
Made important contributions to the prevention and control of severe and major infectious diseases, biological prevention and industrial development in China
Regulations in my country-the main content and relationship of the two regulations
The scope and main content of "General Requirements for Laboratory Biosafety" (GB19489)
Software: Laboratory Biosafety Management-Biosafety Classification, Laboratory Safety Behavior
Hardware: Principles of Biosafety Laboratory Construction-Laboratory Facilities, Equipment, Personal Protection
The scope and main content of the "Technical Code for Building Biosafety Laboratory" (GB50346)
Scope: suitable for microbiology, biomedicine, animal experiments, genetic recombination and biological products, etc.
Content: Design, construction and acceptance of biosafety laboratories such as new construction, reconstruction and expansion
The relationship between the two norms
GB19489 is the basis and process requirements for the preparation of GB50346; GB50346 provides hardware guarantee for the realization of GB19489
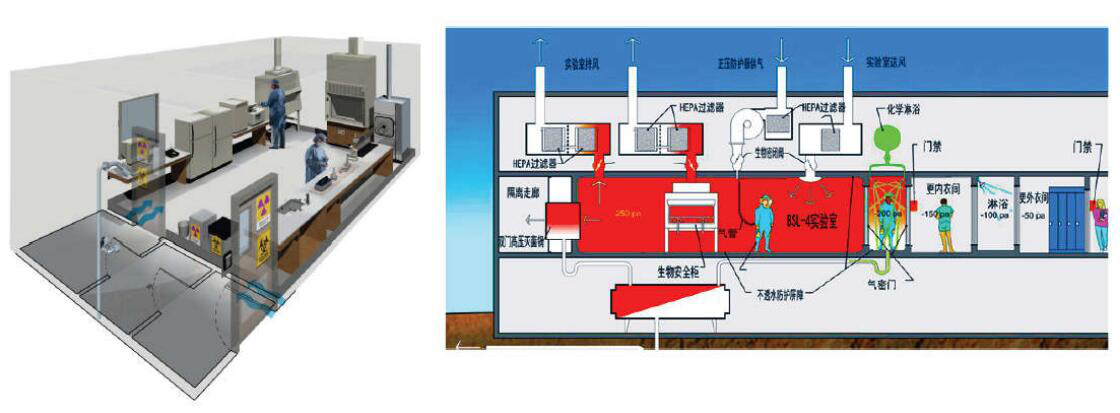
Focus on:
1. The verification process is carried out using instruments or equipment within the validity period of the calibration.
2. The number of air changes should meet the requirements of the specification and ensure that the air volume and pressure are within the working range of the regulating valve.
3. Pay attention to maintaining the tightness of the structure to ensure the cleanliness of the indoor environment under absolute negative pressure.
4. Familiar with the scope of A/BSL protection zone to ensure that the pressure gradient meets the specification requirements.
5. Ensure that the fan interlocking and fault output logic are correct to avoid the appearance of absolute positive pressure.
6. The pressure decay method should pay attention to temperature changes during the air tightness test.
7. The reliability verification process should be carried out under the operating conditions of key equipment.